Illusion Systems
Patently O
NOVEMBER 20, 2022
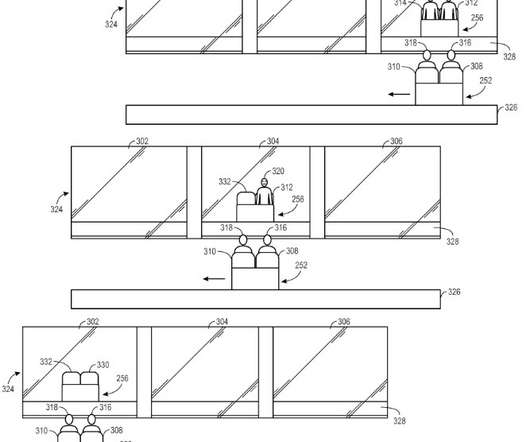
Mike Brister is a theme park designer and I love his 2022 patent directed to a “vanishing illusion system.” The basic idea is that a portion of the glass is reflective while other portions are transparent. Patent examiner issued a first-action allowance on the following claim: 1. ” U.S. 11,235,258 .





















Let's personalize your content